(通訊員:朱碩)近日���,華中農業(yè)大學動物科學技術與動物醫(yī)學院曹勝波教授����、葉靜教授團隊在Journal of Neuroinflammation雜志上發(fā)表了題為“H3K27me3 of Rnf19a promotes neuroinflammatory response during Japanese encephalitis virus infection”的研究論文�。該研究首次發(fā)現(xiàn)了表觀遺傳修飾在日本腦炎病毒誘導的神經炎癥中發(fā)揮重要作用,闡明了Rnf19a調控日本腦炎病毒誘導神經炎癥反應的分子機制�����,為尋找日本腦炎治療藥物靶點提供新的線索��。
日本腦炎是由日本腦炎病毒(Japanese encephalitis virus infection, JEV)感染引起的蚊媒傳播人畜共患傳染病��,該病主要引起種豬的繁殖障礙�,導致母豬流產,公豬睪丸炎�����,對我國養(yǎng)殖業(yè)造成巨大經濟損失��。同時JEV還可由豬經蚊向人傳播���,引起人神經系統(tǒng)疾病��,嚴重可造成致死性腦炎��,但該病毒引起中樞神經系統(tǒng)炎癥反應的分子機制仍有待闡明����。
研究人員首先發(fā)現(xiàn)JEV感染會導致小鼠小膠質細胞系BV2、原代膠質細胞和小鼠腦組織中H3K27me3水平明顯上調����,并利用甲基化轉移酶EZH2抑制劑證明H3K27me3修飾可能在JEV誘導的小膠質細胞炎癥中發(fā)揮重要的調控作用。

圖1. JEV感染引起小鼠小膠質細胞系BV2�����、原代膠質細胞和小鼠腦組織中H3K27me3水平上調
隨后����,研究人員利用ChIP-Sequencing分析JEV感染細胞中H3K27me3所參與調控的基因,最終篩選出JEV感染后炎癥抑制性基因Rnf19a啟動子區(qū)域對應的H3K27me3修飾明顯上調��,而Rnf19a表達量明顯下降���。隨后通過使用基因敲除和過表達手段�,證明了Rnf19a在JEV誘導的小膠質細胞炎癥反應中起負調控作用。進一步實驗證明E3泛素連接酶Rnf19a是通過泛素化降解RIG-I從而抑制炎癥反應���。以上研究結果表明JEV可通過上調Rnf19a啟動子區(qū)域H3K27me3修飾�����,抑制該基因表達,從而促進炎癥反應的發(fā)生����。
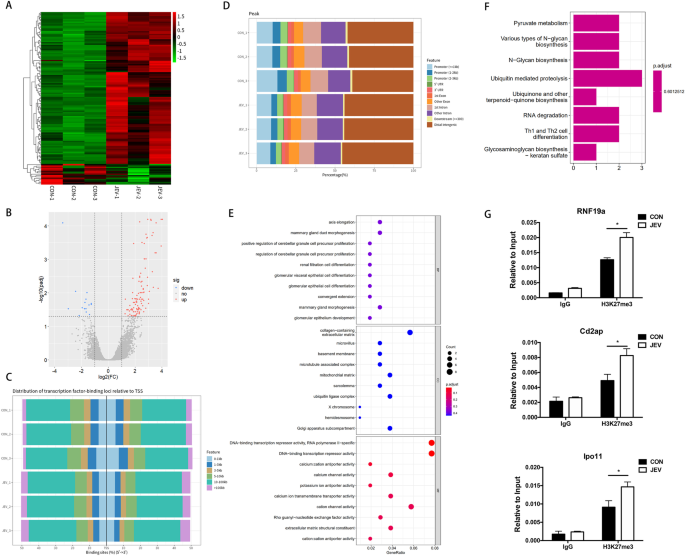
圖2.ChIP-Sequencing分析JEV感染細胞中H3K27me3參與調控的基因
我校動科動醫(yī)學院研究生朱碩和陶夢穎為論文共同第一作者,葉靜教授為論文通訊作者���。該研究得到國家自然科學基金���、國家重點研發(fā)計劃項目等項目資助。
【英文摘要】
Histone methylation is an important epigenetic modification that affects various biological processes, including the inflammatory response. In this study, we found that infection with Japanese encephalitis virus (JEV) leads to an increase in H3K27me3 in BV2 microglial cell line, primary mouse microglia and mouse brain. Inhibition of H3K27me3 modification through EZH2 knockdown and treatment with EZH2 inhibitor significantly reduces the production of pro-inflammatory cytokines during JEV infection, which suggests that H3K27me3 modification plays a crucial role in the neuroinflammatory response caused by JEV infection.The chromatin immunoprecipitation-sequencing (ChIP-sequencing) assay revealed an increase in H3K27me3 modification of E3 ubiquitin ligases Rnf19a following JEV infection, which leads to downregulation of Rnf19a expression. Furthermore, the results showed that Rnf19a negatively regulates the neuroinflammatory response induced by JEV. This is achieved through the degradation of RIG-I by mediating its ubiquitination. In conclusion, our findings reveal a novel mechanism by which JEV triggers extensive neuroinflammation from an epigenetic perspective.
原文連接:https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-023-02852-4
審核人:葉靜
